Read about some of our recent work on understanding the genetics of kidney disease.
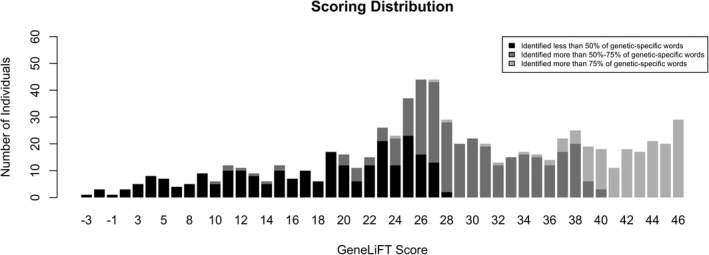
Distribution of the GeneLiFT score. Black represents those who recognized less than 50% of the genetic‐specific words, dark grey represents those who recognized 50%–75% of the genetic‐specific words, and light grey those who recognized more than 75% of the genetic‐specific words. Low genetic literacy was assigned to those with a score of 23 points or less. The mean score in this cohort was 28.3 points and the median was 28 points.
June, 2021
With the broader introduction of genomic medicine in research and clinical care, an increasing number of persons are offered genetic testing. Many factors, including genetic literacy, may impact the utilization of genetic results by patients and their families. We developed a rapid, self-administered measure of genetic literacy, called Genetic Literacy Fast Test (GeneLiFT). We next evaluated the association of GeneLiFT scores with the comprehension of limitations of genomic medicine in participants undergoing genetic testing in the NIH-sponsored eMERGE III study at Columbia University Irving Medical Center, New York. All participants underwent genetic screening for variants in 74 actionable genes associated with adult-onset disorders. A diverse cohort of 724 participants completed the survey (60% women, 45% less than 40 years old, and 53% self-reported White non-Hispanic ancestry). The GeneLiFT was validated using known group differences based on education, health literacy, and numeracy, and with questions assessing genetic knowledge. GeneLiFT identified multiple standard genetics terms, that is, jargon, not recognized by more than 50% of participants (including actionability and pathogenicity). Low genetic literacy, identified in 210 participants (29%), was significantly associated with poor understanding of the limitations of genetic testing (p-values < 10-9 ). This association was independent of education, health literacy, and numeracy levels, highlighting the importance of directly measuring genetic literacy. Low genetic literacy was also associated with low satisfaction with the informed consent process. GeneLiFT is a practical tool for rapid assessment of genetic literacy in large studies or clinical care. GeneLiFT will allow future research to efficiently assess the role of genetic literacy on the clinical impact of genetic testing.
Read the Article in the J Genet Couns. 2021
Feb 17, 2021
Vesicoureteral reflux (VUR), the retrograde flow of urine from the bladder towards the kidneys, caused by malfunction at the vesicoureteral junction is associated with progressive renal disease and is a common cause of febrile urinary tract infection (UTI) and pediatric kidney failure. VUR has a prevalence of 1-2% in European populations Familial aggregation, with reported occurrence rate of 27-51% among siblings and 66% among offspring of affected individuals, supports a hereditary basis. We conducted the largest VUR copy number variant analysis (CNV) and genome-wide association study (GWAS) to-date, accounting for multiple modes of inheritance and sex- specific effects in VUR. Our dataset included participants from major national cohorts such as RIVUR and CKiD.
We found that 3% of patients with VUR have an unsuspected genomic disorder that is not clinically recognized, with significant clinical implications given the relatively high prevalence of VUR in the pediatric population.
The GWAS for VUR identified loci with large effect, encompassing genes that participate in embryonic development (WDPCP, BMP5, WNT5A and VANGL1). Our detailed studies of Wnt5a mutant mice showed a novel role for WNT5A during early stages of bladder and urethral formation.
Altogether, in our study, about 7% of VUR cases carried high risk genotypes, defined as major CNV disorders (3%) or homozygosity for the WDPCP risk allele (4%).
Read the Article in the JASN
May 16, 2019
Kidney transplantation is the best treatment for end-stage kidney failure, but rejection by the recipient’s immune system remains a major problem. In collaboration with the Kiryluk lab, we studied whether genetic mismatch between donor and recipient can explain kidney transplant rejection.
There are over 150,000 people leaving with a kidney transplant in the United States. Moreover, kidneys are a scarce resource so we must make sure that all transplanted kidneys have the best chance of functioning for a long time. Donors are usually matched to a recipient based on blood type and genotype at the HLA (histocompatibility locus). In our project, we asked whether incompatibilities in other regions in the genome explain some transplant rejections. We searched for situations where recipient harbors a common copy-number variants (CNVs) that may disrupt a gene such that the recipient’s immune system has not encountered the encoded protein before. In such situation, the recipient’s immune system would be predicted to mount a rejection response towards a kidney that normally expresses the protein.
Investigating this hypothesis in 2700 transplant recipients and found that recipients who are homozygous for a CMV downstream of LIMS1, have in a nearly 2-fold increased risk of rejection, particularly when they receive a kidney from someone who is not homozygous for the CNV. We also found that these individuals mount an antibody response to LIMS1, presumably leading to injury in the transplanted kidney. This situation is expected to impact about 15% of transplanted kidneys and would be preventable if we determine genotype for the LIMS1 CNV before transplantation to avoid a “collision” of genotypes. Thus, our study uncovered a new histocompatibility locus, outside the HLA regions, which mediates risk of transplant and offers a new tool for improving donors and recipients-matching and subsequent kidney transplant outcomes.
Read the news article in the CUIMC Newsroom | Article in the NEJM
January 10, 2019
Exome sequencing (ES) is quickly emerging as a first-line diagnostic tool in clinical medicine, but its utility has not been investigated for the majority of constitutional disorders in adults, including for chronic kidney disease (CKD), which collectively affects more than 1 in 10 individuals globally. Thus, we performed ES in a combined cohort of 3,315 ethnically diverse patients with all-cause CKD, 91.6% of whom were adults, reflecting the demographics of the greater CKD patient population.
We detected diagnostic genetic variants in 9.3% of cases, a rate similar to that observed for all-cause cancer, where ES is routinely used. On case-level review, we found that genetic diagnoses would meaningfully shape medical care, including informing prognosis, initiating subspecialty referral and/or influencing the choice of therapy. In addition, we identified mutations for other medically actionable findings in 1.6% of the patients assessed; though not explicative of patient's CKD, these striking could also impact renal care in all cases. Altogether, this study supports the diagnostic value of ES in nephrology, and also highlights the potential of both primary and secondary genetic findings to inform medical care, including initiating multidisciplinary care and directing patients to disease-specific clinical trials and targeted therapies.
Article in the NEJM
January 1, 2019
Using exome sequencing data of a large cohort of 7,974 unrelated self-declared healthy adults, we demonstrated the potential high burden of widespread reporting of incidental genetic findings related to kidney and genitourinary disorders. This research illustrated the importance of stringent curation of clinical variant databases. In the absence of detailed review, both at the patient clinical level and at the variant level, there is a significant risk of genetic misdiagnosis and unnecessary referrals.
Article in Annals of Internal Medicine
January 2, 2018
In the past few years, we have learned a lot about the genetics of kidney and urinary tract malformations. In this paper, we review recent advances in the field and discuss opportunities and challenges ahead.
Article in Annals of Internal Medicine