CAKUT accounts for 40-50% of pediatric end-stage kidney failure worldwide.
Overall, data indicate that ~20% of patients may have a genetic disorder that is usually not detected based on standard clinical evaluation, implicating many different mutational mechanisms and pathogenic pathways. Application of microarrays has shown that 10-15% of CAKUT patients harbor an unsuspected genomic disorder that explain many disease comorbidities such as neurocognitive impairment, and whose early recognition can impact clinical care.
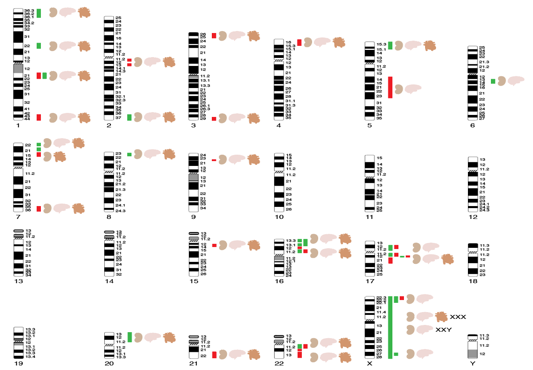
Map of CNVs predisposing to CAKUT, congenital heart disease and impaired neurocognition
(Sanna-Cherchi et al, JCI 2018)
In parallel, using exome sequencing, our lab led to the discovery of new CAKUT genes DSTYK, GREB1L, NR6A1 and ARID3A , implicating new pathways in disease pathogenesis. We have also contributed to the discovery of ZMYM2, NRIP1, TBX18, XPO1, and WBP11 as new CAKUT genes, and TBX6 and CRKL as drivers of CAKUT in the 16p112 and 22q11.2 syndromes. We continue to apply high-throughput genomic technologies to large cohorts of patients gain insight into the common and rare genetic determinants of diseases and identify opportunities for early diagnosis and personalized care.
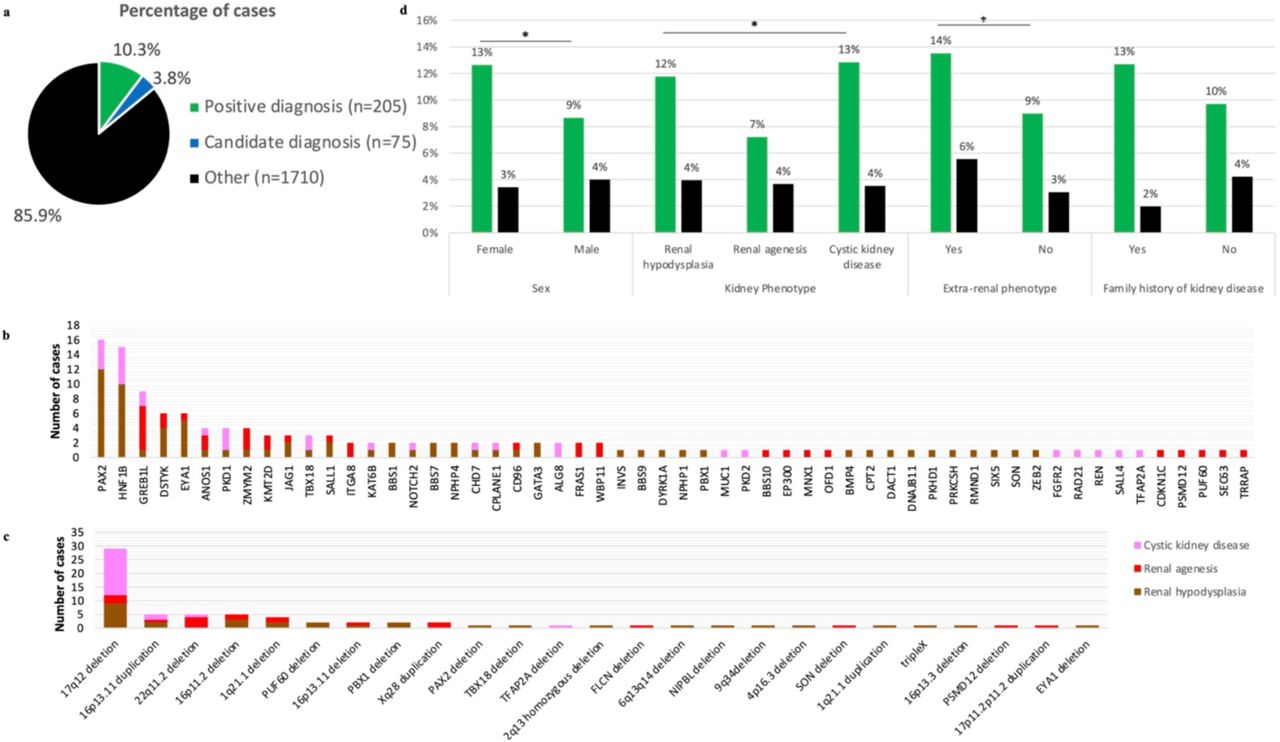
Diagnostic analysis in 1,990 unrelated probands with kidney anomalies
(Milo Rasouly et al, medRxiv 2024)
Recent advances in genome sequencing and interpretation have provided fundamental insights into many medically important diseases, allowing for reclassification based on primary molecular pathogenesis and enabling more precise surveillance, therapy, prognosis, and counseling. While these scientific advances have led to widespread enthusiasm about the potential of genomics to transform clinical care, many challenges still need to be resolved before cost-effective clinical sequencing becomes routine. Challenging areas include correct and consistent interpretation of genomic findings; acquisition and interpretation of the data in a clinically relevant timeframe; development of reporting practices that patients and providers are able to understand and utilize to make decisions; and demonstration that clinical sequencing improves outcomes. In ongoing studies, we have shown that genetic testing can change clinical management, including choice of therapy. In partnership with etc National Kidney Foundation, we also help develop the first guideline for genetic testing for kidney diseases.